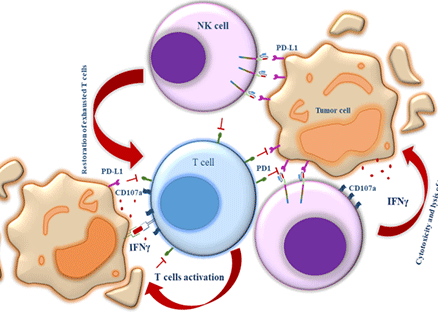
Strong capacity of differentiated PD‑L1 CAR‑modified UCB‑CD34+ cells and PD‑L1 CAR‑modified UCB‑CD34+‑derived NK cells in killing target cells and restoration of the anti‑tumor function of PD‑1‑high exhausted T Cells
16 November 2024
174 Views
No Comments